Celiac Disease, the Microbiome, and the Gluten Intolerance Spectrum
It's not as simple as "you have it or you don't".
Meta: We’re on month five of this blog’s existence, and starting to regularly break into the 500+ views per post. Many thanks to all the loyal readers, and a warm welcome to all the new ones! When we hit a thousand subscribers I’ll probably introduce “Certified Shithead” merch of some kind. The multiple sclerosis post got a lot of love, so I figured this week we’d do another radical hypothesis—this one is for my dear friend Mimi, who lives, as the Italians say, senza glutine.
There’s a certain crowd of people who I often find myself at odds with. Let’s call them science enthusiasts. They’re sometimes actual scientists, but more often than not they’re just nerds—and really, most scientists are so specialized in our various fields that there’s no functional difference when it comes to a topic outside of it. Science enthusiasts are the type of person who will occasionally, from a position of well-meaning self-righteousness, admonish heretics to #believethescience—seemingly forgetting that science is not a body of knowledge, but a constant process of guesswork and revision, where nothing is ever set in stone.
Science enthusiasts love to hate on the “gluten intolerant”.
Gluten intolerance doesn’t exist, they’ll say. Celiac is real, but if you don’t have it, you’re just a baby with an upset tummy who gets off on making life difficult for the kitchen staff. (This, in spite of the fact that there’s now an ICD-10 code for non-celiac gluten intolerance.)
Among the less hidebound, you’ll find all manner of explanations for gluten sensitivity. Some claim that it’s actually a sensitivity to the glyphosate that’s sprayed on wheat, and—while I am suspicious of glyphosate on some fronts, I don’t think that’s a fit here. For one, it’s used in much greater abundance on corn, soybeans, and sugarcane, but something about wheat specifically trips up a lot of people who do more-or-less fine with soy. For another, glyphosate is light enough to distill out into liquor, so the fact that gluten-intolerant people can usually enjoy a shot of Tito’s without getting a flare-up makes me think there’s something else going on here.
The Whodunit
So let’s start with the obvious: what is celiac?
Well, we don’t really know. The orthodox consensus seems to go as far as “idiopathic autoimmune disease”,1 and that it’s caused by an inflammatory reaction to gliadin, a component of gluten—but that’s a what, not a why.
People have looked in the genome, and while there are gene variants that are pretty much necessary for a person to have celiac (95% of people with celiac have a certain allele of a gene called HLA-DQB1) they’re not sufficient: even among people who get “bad” versions of this gene from both parents, only ~30% will go on to develop celiac. The timing of the disease also doesn’t really fit with that of a genetic disorder, either, because true genetic disorders are typically something you have from birth. While celiac often manifests early on, it can crop up at any point in life.
So why do only a portion of genetically susceptible people end up with celiac? What is it that’s activating the latent risk factors in them? Or is there something protecting the other 70%?
When learning about an unsolved disease, I have a little mental checklist for figuring out whether there’s likely to be a microbiome component to it, which looks something like:
Mysterious etiology?
Autoimmune/inflammatory aspects?
Risk genes, but no sufficient gene in most cases?
Emerges well after birth, but then tends to stick around?
Broad spectrum of severity?
GI symptoms?
Celiac ticks all these boxes except #5—and even that one is tricky. Diagnosis often comes down to the opinion of the pathologist looking at your intestinal biopsy under the microscope, and someone on the road to celiac—but who gets tested before the villi of their small intestine have started visibly wasting away—might later get retested and find out that now they have celiac, where before they just hadn’t made the diagnostic cutoff yet.2
A Quick Primer on Gluten
Before we go on, it’s worth taking a second to give a profile on our main culprit in this case: gluten.
Gluten isn’t a singular substance, because the stuff was named by bakers and pasta-makers, not molecular biologists. There are two main components to gluten, both of which are proteins—long strings of amino acids. One is glutenin, the chains of which are long and interlinked with one another to form a kind of mesh. These chains take on a coiled, kinked shape at rest, but they can stretch under tension, straightening out without breaking. In a very real sense, glutenin is the molecular spring that gives bread its…well…springiness.
The other component is gliadin: this is the “bad” stuff. Chemically, it’s not that different from glutenin—although it’s smaller. (I say “smaller” but, while all of these things are microscopic, each gliadin molecule is still over a thousand amino acids long.) Unlike glutenins, gliadin molecules don’t link together with other gliadins: they tend to bunch up by themselves, interspersed among the long glutenin tangles. Both of these are storage proteins for the seed: gluten is how the wheat plant banks up spare amino acids, to give its offspring everything it needs to get the jump on life. A kind of college fund.
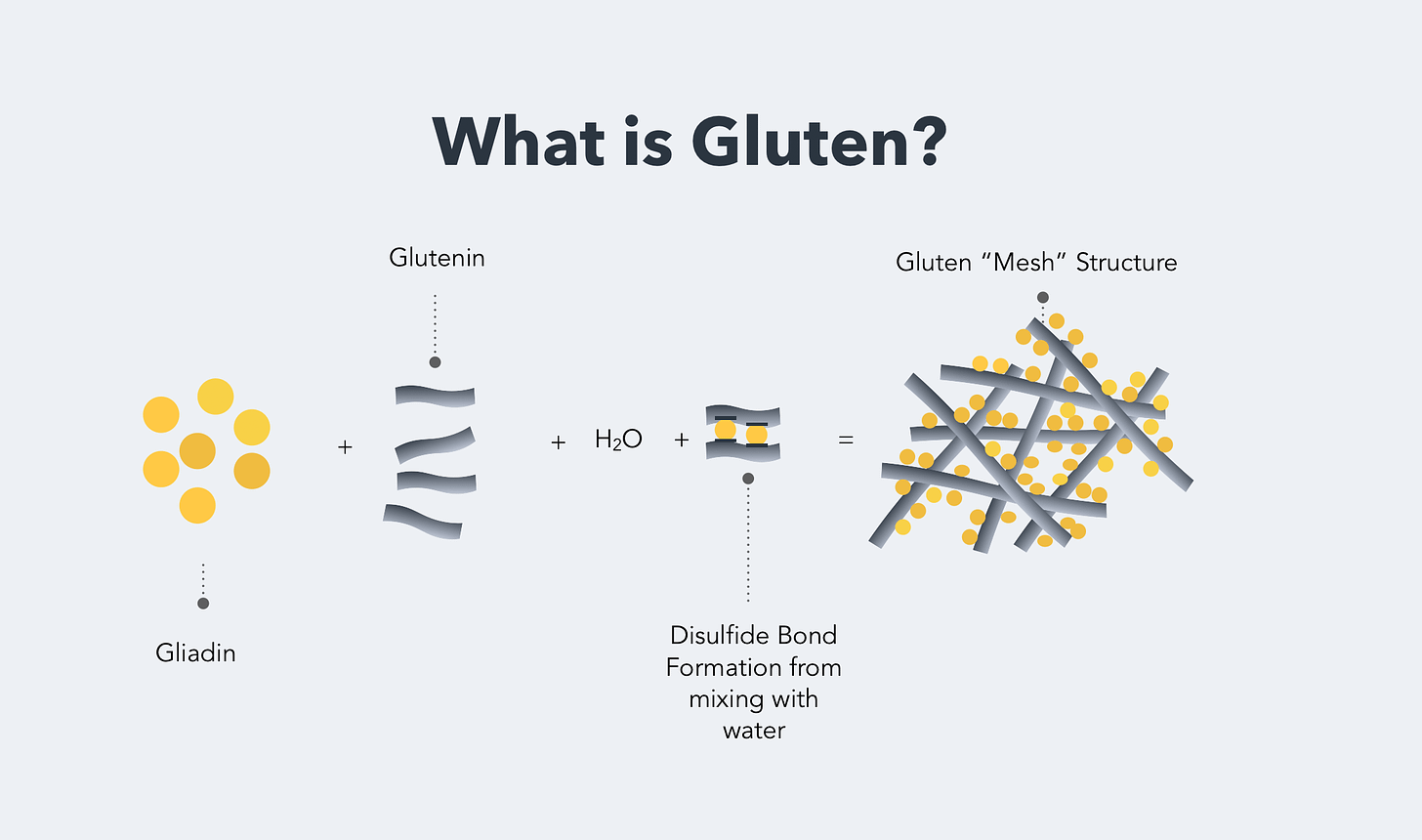
The conventional wisdom is that the body of a celiac patient can’t digest gliadin properly, and the undigested fragments trigger the immune response. But there’s more to the story.
What’s a Hapten?
Not much, whassa hapten with you?
Sorry. A hapten is a molecule which, floating around by itself, doesn’t cause problems—but which has a tendency to stick to proteins or other molecules near the surface of a cell. This can cause an autoimmune reaction, because a lot of cell-surface molecules act like the flags on a ship at sea, broadcasting information about their origin and purpose to other cells. When a hapten sticks to a cell, your immune system sees a flag it doesn’t recognize…and opens fire on the apparently foreign invader.
It’s been proposed that celiac disease is a hapten-like response to undigested bits of the gliadin molecule: these fragments can stick to an enzyme called tissue transglutaminase. If you have the right genotype, your immune cells can pick this fragment up, match it the hapten-modified enzyme, and start wrecking shit.
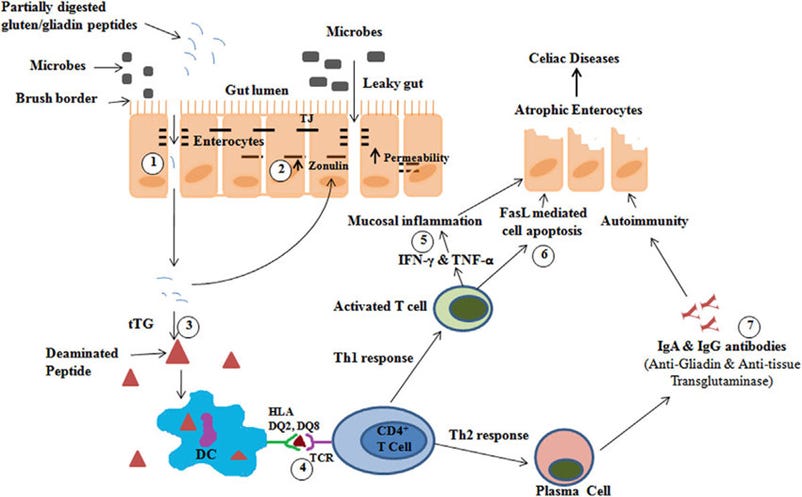
But here again, this is a what, not a why. That diagram glosses over an important question: it starts in the upper left with “partially digested gliadin peptides”—but why can’t celiacs digest gliadin?
See, the HLA-DQ gene doesn't code for a digestive protein; it's an immune gene. People have searched and searched, to try and find some digestive enzyme that celiacs are missing—but to no avail. All the machinery seems to be there, working as expected. This is a shame: if it were as simple as a missing enzyme, it’d be a relatively easy thing to supplement that enzyme with meals, a sort of Lactaid for celiac.3
A lot of people have adopted a "leaky gut" theory of the disease, where gut dysbiosis enables gliadin fragments to get into the bloodstream, but this doesn't sit quite right either: for one thing, the antigen-presenting immune cells which express the HLA-DQ complex and mediate the inflammatory response also patrol in the GI tract.
But maybe the reason why we can’t find a good answer to “Why can’t celiacs digest gliadin?” is that we’re asking the wrong question. In 2009, a research article came out in the journal Gut, which proposed a different one.
The title of the paper was “Is It True That Coeliacs Do Not Digest Gliadin?"4 and the findings it reported suggested that the entire field had been looking at the problem backwards. Like everyone else, the authors had gone looking for something that should be there but isn’t: testing tissue biopsies from the guts of celiac patients and controls for their ability to degrade gliadin.
To their surprise, they found something that shouldn’t be there but is. Seven “somethings”, in fact: a cocktail of gliadin-degrading enzymes found in the upper small intestine of nearly all celiac patients, but totally absent in nearly all healthy controls.
The kicker? They’re not human enzymes.
Not very Neiss
This suggests that there’s a pathogenic component to celiac: some organism living in the gut that breaks down gliadin in an unusual way, leaving behind fragments that can act as a hapten or otherwise trigger the immune response. But because the study didn’t use genetic or molecular techniques like protein sequencing, they couldn’t pinpoint the enzymes’ structures or the organism responsible for producing them.
But 2009 was a while ago, so I did a little digging to see if anything had come out on the topic since then—which quickly turned up a more recent paper which sequenced the upper GI of celiac patients, and found unusually high levels of an organism called Neisseria flavescens.
This set off some immediate alarm bells for me. Like a lot of bacteria, there are good Neisseria and bad ones—but the bad ones are pretty nasty. Neisseria meningitidis causes bacterial meningitis, an often-fatal infection of the soft tissues around the brain and spinal cord, while Neisseria gonorrhoeae causes the STD gonorrhea. A quick dive into BV-BRC, an enormous database of microbial genomic information, revealed a variety of poorly-characterized protease enzymes in Neisseria flavescens that seem, at first glance, to fit the bill.
Most people have a few Neisseria in their GI tract; species like Neisseria lactamica are often a normal component of the microbiome in the mouth and duodenum (just past the stomach), where they likely play a role in helping prevent colonization by more pathogenic strains. This, and the fact that there are genes which are necessary but not sufficient for celiac to emerge, suggests a three-factor model of celiac, where it’s caused by an organism that’s only a pathogen if you’ve got the right genes, and even then only if you’re eating gluten.
If this is the case, we’d naturally expect a spectrum of gluten sensitivity to emerge among people with the right genes, because bacteria can exist at any level of abundance: someone whose microbiome plays host mostly to N. lactamica, but with a minor population of N. flavescens, might have a mild gluten sensitivity that’s still fundamentally the same as celiac disease.
This is exciting, because it suggests some relatively simple solutions to celiac. Antibiotics are a theoretical possibility, but seem like a risky approach, since there’s no chemical that can kill Neisseria without damaging the rest of the microbiome and potentially causing a bunch of other problems.
But finding a bacteriophage that kills the offending strain might be all it takes to cure a person. Phages are very specific to the type of bacteria they infect, and this is both a blessing and a curse: it minimizes collateral damage, but strain-level variation among patients means that there’s no guarantee a phage which works for one person’s N. flavescens would work for another’s. Typically, you have to isolate the microbe from the individual you’re trying to treat, find a phage that kills it, and then feed that phage to them. This is why phage therapy is still pretty rarely used: it’s hard to scale. Even so, Neisseria is so abundant in the mouths of celiac patients that it can usually be isolated from a single throat swab, which would lend itself to easy development of a personalized therapy.
It might also be possible to out-compete and supplant the pathogenic strain with one like Neisseria lactamica, although this approach seems risky, because Neisseria are really good at horizontal gene transfer. That means that if you feed a nonproblematic strain to someone already colonized by a problematic strain, you’d risk the “good” strain simply picking up those problematic gliadin-degrading enzymes and becoming a “bad” one.
There are two more facts which, taken together with all the above, paint a really interesting picture of the disease’s origin at a global scale. One is that Neisseria is pretty much a human-exclusive genus, and the other is that the risk genes are only common among certain ethnic groups.
To me, this suggests that—if a strain of N. flavescens is really the causative agent in celiac—it must have evolved as a commensal in an ethnic group where those susceptibility genes are rare, where it wouldn’t have caused problems—or else in a culture where staple crops didn’t contain gluten, like South America or east Asia.
When I explained this hypothesis to my friend Greg, he asked if the presence of Neisseria in the mouth would mean that you could catch celiac from kissing someone with the disease. An understandable concern, since Greg kisses a lot of people. I told him it depends: if you don’t have the risk genes, you’d probably be in the clear—but if you do, it’d be a theoretical possibility in this model. What’s more, this would imply that a “carrier”, who has the strain but not the genes, could potentially infect someone with the risk genes and cause celiac in them.
In this model, celiac is a disease of modernity. As we transition into a globe-spanning society, the roiling currents of intermingling cultures, genotypes, and microbiomes create new new variations on the theme of human biology: new combinations of host, microbe, and diet. People meet and marry outside the genetic siloes of their ethnic groups, and recessive diseases like Tay-Sachs begin to disappear. But at the same time, we create the potential for exposure to pathogens that our immune systems aren’t prepared for—like smallpox in the Americas.
In a sense, it’s the unstoppable wave of technological advancement that’s driven this cultural and biological convection, accelerating trade, travel, and communication. But where this has created wrinkles, it’s also given us the ability to smooth them out: The beginning of the end of smallpox in America was when a man called Onesimus, enslaved and taken from Africa as a child, brought with him his culture’s knowledge of variolation—a rudimentary form of vaccination.
A little under three hundred years later, a disease which had plagued humanity for millennia has been eradicated, but it’s all too easy to imagine a world where Onesimus was ignored by his enslaver, and the insight was squandered.
Nowdays, in an age where the internet provides instant access to a staggering body of scientific and medical knowledge, it’s easier than ever to find the information that we need to solve our biggest problems. There’s a genuine possibility that, for a lot of them, we already have all the pieces we’d need to put the puzzle together. In these cases, the only problem is one of utilization: connecting dots, applying existing knowledge, and getting the right people to listen. I think how well we’re doing as a society can be measured largely by how efficiently we’re using our newfound potential to solve the problems we’ve inadvertently created: how rapidly we can recognize those solutions when they come to us, and use the infrastructure we’ve built to deploy them at scale. I think we’ve got some work to do.
🖖🏼💩
A series of words which makes the eyes roll back in my head because it means next to nothing
This is what I call a “mayonnaise problem”: there’s no such thing as a really amazing mayonnaise, because as soon as you start adding things like spices and garlic, it stops being mayonnaise and starts being aioli or remoulade or something. Likewise, there’s no “mild celiac”, because the problem has to be really bad to be called celiac.
The real tragedy is that we could have had “celiactaid”.
Question mark in the title of a paper: usually a big red flag, but less so when it’s reporting clinical experimental results.



As someone with mild gluten intolerance, I find that with bread and pasta a slow overnight sourdough ferment makes these foods edible. Perhaps the LAB and non- S. cerevisiae yeast can metabolize proteins other than gluten that might be the route cause of my issue. (Can't be gluten as sourdough rises, and I tested by adding gluten flour to bread). I've heard other people with an intolerance finding sourdough digestible.
Glyphosate is used to desiccate wheat at the end of harvest. It increases the shikimic acid content which is used in the production of tamiflu.