A Strange and Wonderful Beast, Pt. I
An informal report on the isolation and characterization of *Candidatus Astrobacillus crystallinus,* (gen. nov, sp. nov.) a cholesterol-reducing bacterium from the human gut.
Imagine being woken in the predawn light, at the break of some prehistoric day, by the sound of a friend saying your name. You open your eyes, and he’s standing there, staring across the plains into the distance.
“Get up,” he says, not turning his head.
You follow his gaze. After a moment, dim shapes on the horizon resolve: a herd of wild horses.
As you rise to your feet, he turns to you, and now you can see there’s a dangerous glint in his eye. A wild, insane look, with a vision behind it. One of glory, of riding on thundering hooves.
In his hands, he’s clutching a woven rope.
“Come on,” he says. “I’ve got an idea.”
That’s where we are right now. It’s gonna be a big day.
When I wrote those words two and a half years ago, I read it back and thought “ehh, it’s a bit much, isn’t it? Am I being melodramatic?”
But friends, I come before you today a man triumphant: one who set out to wrangle horses, and instead comes home riding a unicorn. A strange and wonderful beast, known from tales in days long past (think, like, 1980), but which has eluded capture for so long that it had started to take on the air of legend. Ladies & gentlemen, it’s my great honor to introduce: the human gut’s native cholesterol-reducer, a microbe much-discussed but never before seen in the flesh…until now. A bacterium that might just save your life.
I’m calling it Astrobacillus, the star-rod.
Cholesterol and Coprostanol: A Quick Review
If you’re a longtime reader, you might remember this piece on cholesterol from back in 2023. For those of you just joining us, the gist is that humans (and all mammals, really) are supposed to have gut bacteria which help us get rid of excess cholesterol.
It’s an important job. On top of the cholesterol in your diet, your liver makes nearly a gram of the stuff each day, to help you absorb nutrients in your food. If it doesn’t leave the body as part of your poop, it can build up in the bloodstream in the form of atherosclerotic plaque. When a chunk of this stuff breaks loose and clogs an important blood vessel, you’ve got a heart attack or a stroke on your hands.
So we rely on these gut bacteria, which enable us to shit out cholesterol by transforming it into a chemical called coprostanol. See, left to its own devices, the body recycles cholesterol very efficiently, secreting it into the gut and then taking it back into the bloodstream along with the fats in your food. The transformation into coprostanol (a reduction reaction) causes it to precipitate, to crystallize in the GI tract, preventing its reabsorption: out of the loop, ‘til it’s out in the poop.
But in the ‘70s and ‘80s, it was discovered that roughly 1 in 5 people are missing this pathway, and have little or no coprostanol in their stool. In the ‘90s, we learned the likely reason why: a standard week-long course of oral antibiotics has about a 10% chance of driving the coprostanol-producers in your microbiome extinct—meaning you basically lose the ability to shit out cholesterol. Hopefully it’s not hard to see how this could become a problem.
This finding is exciting, because it suggests that the root cause of a lot of cases of hypercholesterolemia could be as simple as a lack of coprostanol-producing bacteria. If we can put them back, maybe we can cure high cholesterol.
The only catch? Nobody had ever isolated a coprostanol producer from the human gut before. They’re considered “uncultured”.
In the ‘80s, a handful of coprostanol-producing strains were isolated from the guts of baboons and rodents, but all of these were somehow “lost”, according to the literature. More detail than that is mysteriously hard to find, but these things happen all the time—a freezer fails, or a lab floods, or a scientist dies and nobody picks up the project they were working on.
In 1994, one strain was isolated from a “hog sewage lagoon”,1 and this organism (dubbed Eubacterium coprostanoligenes HL) is—to date—the only confirmed coprostanol-producing bacterium known to man.
But that’s about to change.
Materials and Methods
Isolating this guy wasn’t exactly easy, but it also didn’t require any great leaps of insight; just diligent research (shoutout to Alexandra Elbakyan at Sci-hub), an anaerobic lab (shoutout to Raja Dhir at Seed), and a willingness to do some things that, to your average person, probably look insane.
See, when I wrote that first piece on cholesterol and coprostanol, I had already found the papers from the ‘70s where they described isolating the first coprostanol-producing bacteria in liquid culture—a painstaking process of serial dilutions and antibiotic treatments, because the organism refused to grow on solid agar in a petri dish.2 What I hadn’t yet found when I wrote that post was an article by Brinkley, Gottesman, & Mott (1980)—a paper from just a few years later, where they tell you exactly how to make an agar that coprostanol-producing bacteria will grow on.
The secret ingredient is “way more cholesterol than seems reasonable”…and a healthy helping of mammalian brain.
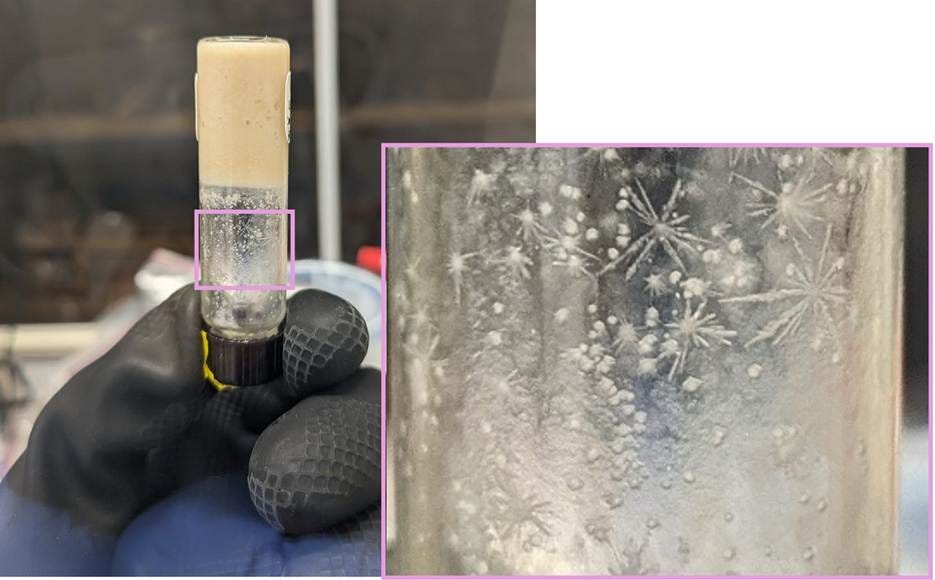
But once the brain has been freeze-dried and papain-treated, and the special agar has been made, nabbing one of these bacteria was as simple as taking a shit, diluting it by a factor of ten million or so, spreading a little of that water on the petri dish, and—a week later—looking for the “fibrous” morphology described in the paper.

A Little TLC
The fact that this bacterium was a visual match to the cholesterol-reducers described in the literature was a good start. Sequencing its 16S gene—sort of the bacterial “barcode”—revealed that it wasn’t very closely related to any named organism, but one of the closest was Eubacterium coprostanoligenes. This is even better, but it’s still what a lawyer would call circumstantial evidence. I wanted proof-positive.
Unfortunately, I’m not a very good chemist, and I didn’t have ready access to the kind of equipment typically used to interrogate an organism’s molecular makeup. The Swiss Institute for Allergy and Asthma research—where I was when I first isolated one of these guys—has plenty of mass spec rigs, but they’re all set up for proteomics, i.e. looking at things thousands of times the mass of individual molecules like cholesterol and coprostanol. Lucky for me, back in 1987 some folks at the FDA published a low-tech method that turned out to be just what I needed: cheap, easy, and hard to fuck up.
See, “is this a piece of poop or not?” is a million-dollar question when you find a weird chunk in your corn flakes, so the gold standard way to find out is to check for the presence of coprostanol, since it’s abundant in mammalian feces and not really found anyplace else. The method devised at the FDA relies on thin-layer chromatography (TLC), which is about as simple as analytical chemistry gets; if you ever did the coffee-filters-and-marker experiment in school, you’ve got the gist.3 You put your sample on a plate of powdered silica gel (of DO NOT EAT fame), dip the end into a solvent, and let capillary action pull that liquid up against gravity—carrying the chemicals in your sample along with it. Because molecules move up the plate different rates depending on their structures and properties, they show up as distinct spots.
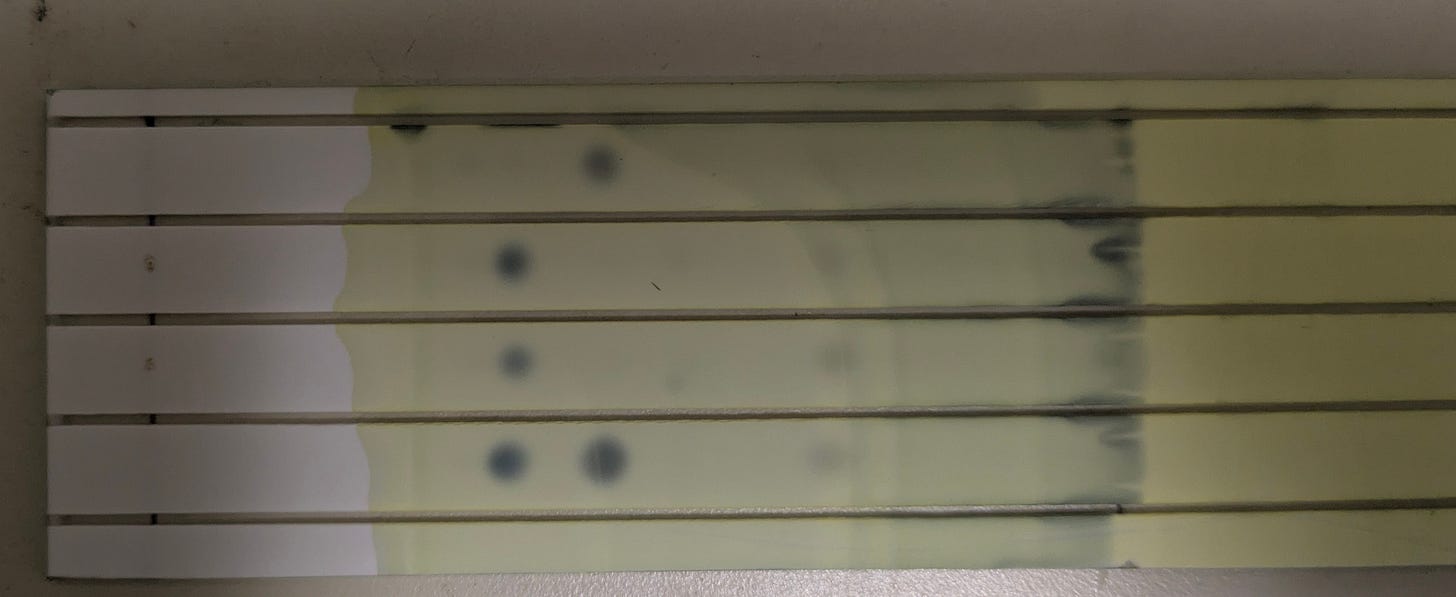
To complete the picture, it helps to have a little context for these dots: that top lane is my own poop sample, and the middle two are my friend Cole’s, from samples taken on different days. I’ve got no cholesterol in my poop, Cole has no coprostanol.
These are two ends of a spectrum; I’ve run this assay on maybe two dozen people so far, and most people have both spots—some people are 90:10, others are 40:60—but my results so far basically agree with the numbers given in the literature: about one in five people are like Cole, in that their microbiome doesn’t seem to produce coprostanol.
But the bottom lane in that image is what you get when you inoculate one of those star-shaped colonies into cholesterol-brain medium and incubate it a few days. The sterile medium starts out looking like those middle lanes—all cholesterol—but by a week later, the coprostanol spot stands out, clear as day.
Gene sequencing the bug reveals that it’s the first isolate of its genus—one of those bacteria that’s currently just denoted by an alphanumeric string wherever it crops up in metagenomics datasets. This is exciting for a couple of reasons, one being that—as the first person to culture one in recent memory—I get to name it. I like the sound of Astrobacillus, for its star shape; we’ll see if that flies with the nomenclature people. (I’m pretty sure it will, but they’ve got a lot of rules and my Latin isn’t great.)
More exciting, though, is having the full genome of this organism, because it acts as a sort of Rosetta stone—turning otherwise-meaningless data into something really useful.
A Brief Foray Into the Weeds
See, over the years, a number of research groups have done coupled metagenomics-metabolomics studies, where they look at the bacterial genes in people’s guts as well as the chemicals in their blood, and search for patterns: for every bacterium X and metabolite Y, you check: is there a correlation between the amounts of these two things in people’s bodies?
And the answer is “yes!” for a lot of X<>Y pairs. Too many, in fact, to even know what to do with most of the information. But recently, someone did a meta-analysis of ten of these studies, and for good measure they threw out any signals that weren’t consistent across at least three datasets. That still left nearly 30,000 microbe<>metabolite pairs, and the researchers were kind enough to publish all that information as a big beautiful excel sheet in their supplemental materials for anyone depraved enough to comb through it. Depending on how much you know about a given metabolite and a given microbe, the info contained in datasets like this can range from “abstruse” to “intriguing yet inscrutable” to “wildly useful”.
For a simple test case, we can look at butyrate: it’s a short-chain fatty acid that’s abundant in the bloodstream, which comes pretty much exclusively from your gut bacteria. You can read about SCFAs anywhere, so for now suffice to say it plays a number of important roles in keeping the body running smoothly.
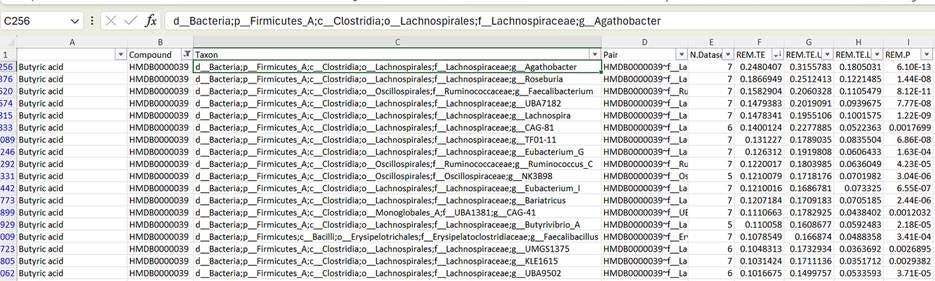
Filtering the table to look exclusively at bacteria that are associated with butyrate, and then sorting by effect size, you can see in the screencap above that bugs like Agathobacter, Roseburia, and Faecalibacterium are the genera that best correlate with butyrate levels in the bloodstream. And this is great—it’s a “pass” on the sanity check: all three of these are known powerhouses for butyrate production, and pretty universally associated with “healthy” status in studies of the gut microbiome.
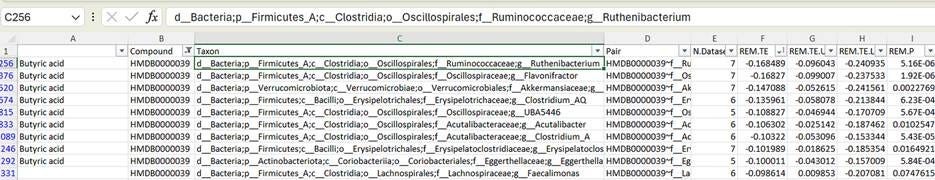
But say we sort the table the other way, and look at inverse associations. What does it mean that Ruthenibacterium is the genus that best predicts low butyrate levels?
Ruthenibacterium isn’t nearly as well-characterized. Does it eat butyrate? Prevent its formation? Or is it an innocent bystander that simply thrives in a low-butyrate microbiome? It’s hard to say, without doing a lot of wet-lab work—and that goes double for things like “UBA5446” on that table: these are the uncultured bacteria. We don’t even know what they eat, or what they turn their food into, and there’s no way to find out for sure, until someone manages to nab one in culture so it can be poked and prodded. If it turns out that the bacterium can grow using pure butyrate as a food source, hey, now we have a pretty good idea as to why it’s associated with low levels of that molecule in the bloodstream.
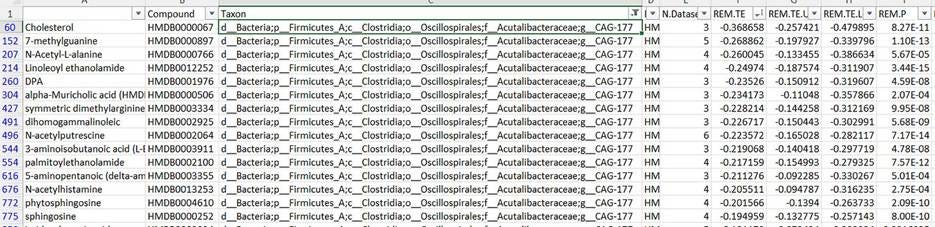
So when I genome-sequenced this coprostanol-producing bacterium, I found out that it shows up as “CAG-177” in tables like this one. And if we do a similar filter-and-sort trick, to see what chemicals it’s associated with in the bloodstream—
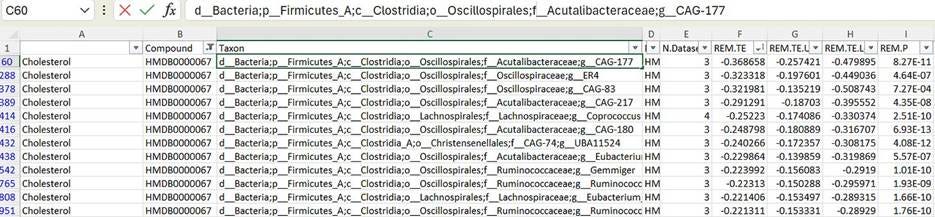
Turns out the abundance of this bacterium in your gut is inversely associated with serum cholesterol levels! You can see that it’s also correlated with a number of other compounds, but of all the chemicals it’s linked to, it’s most strongly tied to cholesterol. Not only that, it turns out this is the strongest association between serum cholesterol levels and any gut bacterium in the table.
In other words: the more of this bacterium you have in your gut, the less cholesterol you tend to have in your bloodstream. Before we knew that CAG-177 was a human-gut-native coprostanol-producer, this was one of those “intriguing yet inscrutable” findings. Now, it becomes solid evidence that coprostanol and the bacteria that produce it are a really important factor in cholesterol homeostasis, and maybe even heart disease risk.
There are still ways this might not be causal in the way that I’m hoping; there’s a world where Astrobacillus only thrives in the gut when your HDL particles are doing a really good job of scrubbing cholesterol out of the bloodstream and depositing it in the GI tract or something. Curiously enough, if you filter-sort the cholesterol x bacteria table the other way—to look at bugs associated with high cholesterol—you’ll find Ruminococcus gnavus at the top of the list. This is a known baddie that I’ll be talking about more in an upcoming post, so I won’t dwell too much on it here—but it’s something I’m chewing on. Maybe one of these bacteria eats the other, or inhibits its growth. Maybe R. gnavus modifies bile acids in a way that prevents your liver from excreting cholesterol into the gut, and this is bad for Astrobacillus abundance. Microbiome science is a kind of ecology, and ecology is always complicated.
Even so, the fact that we’ve found a bacterium that…
a) ought to help lower cholesterol levels, based on everything we know about its molecular biology, AND
b) is associated with low cholesterol levels in multiple actual human cohorts
…is exciting.
Reflections
I struggled for a while to find the right ecological analogy for these guys, but I think that in a lot of respects they’re like the giant panda. Not the fittest, in a lot of senses; slow growing, slow moving, and their milk is so nutrient-poor that—if a mother has twins—she has to pick her favorite and let the other one starve. When all you eat is bamboo, which is about on par with driftwood in terms of nutritional value, it takes a long time to save up enough calories to afford a baby. The flipside is that, when all you eat is bamboo, you never need to worry about where your next meal is coming from. It’s such shitty food that nobody else even bothers with it, so your entire native range is an all-you-can-eat buffet, literally within arm’s reach 24/74. Having exclusive access to an extremely abundant food source goes a long way in making up for whatever you might lack in other domains.
Cholesterol is like bamboo in that, in the microscopic jungle of the gut, it’s extremely abundant. And even more important, a steady supply of it is pretty much guaranteed. Bugs that specialize in the degradation of starch or other complex carbs are subject to the whims of their host’s diet, meaning their population can suffer a serious blow if, say, you go a week without eating a piece of fruit. But regardless of what you’re eating, as long as you’ve got a functioning liver, you’ll have cholesterol in your gut.
There’s lots more to get into here, including some exciting human-subjects-research results to share, but this post is already long enough—stay tuned for the next installment!
—🖖🏼💩
Why nobody has turned that into a probiotic yet, I can only guess. Maybe some university’s lawyer staked out a great patent around it, but didn’t have the juice to do anything more than prevent other people from developing it. Maybe it’s just that, like the one I’ve isolated, it’s fickle and difficult to grow. Or maybe it’s that you could put the entire Anti-Memetics Division of the SCP Foundation to work for a month, and they’d still be hard-pressed to come up with a more mind-erasing, curiosity-averting phrase than “Hog Sewage Lagoon”.
Imagine you’re after a bacterium whose cells represent 1% of the bacteria in a fecal sample. If you want to get it alone, you can dilute the poop down enough that there’s about one bacterial cell per drop of water, and then put a drop into each of 100+ culture tubes and let them grow. This is a pain in the ass compared to agar-based isolation, where you start with poop-water that contains 100 bacterial cells per drop, put one drop on a petri dish, and smear it around so they’re well-separated from one another. Those individual cells turn into visible, “clonal” colonies, which makes it easy to isolate a pure culture.
TLC protocols are use-case-specific—the one that works best for separating the pigments in a dot of black marker probably won’t work for distinguishing cholesterol from coprostanol, but if you’re willing to put in the work to develop a new one, they can be dead useful and very robust.
I like to imagine a very lazy panda who doesn’t even move—just eats a nearby stalk, has a nap, and then eats the same one again when he wakes up and it’s grown back.



Very interesting! Are there any plans to turn this into a probiotic or any live studies to see if the addition of this strain changes the coprostanol composition in waste?
I find it mildly concerning that the ideal feed for this stuff is animal brain matter though. It would be no fun if in return for lowering cholesterol a bit it consumed your brain.
This is fantastic! I am very much looking forward to seeing your work in the future.