There’s this common thing in fantasy or horror literature—I think H.P. Lovecraft really started it—where spooky elder gods, demons, and creatures of the void, etc. have names that are really hard to pronounce. They’ll have unhinged combinations of consonants all in a row, with apostrophes in preposterous places, like Rl’hyerg’x.
The implication is that the language in which these things were named is spoken by beings that are Not Like Us. Their mouths have a few non-standard features…maybe a convertible roof, by the sound of it.
Part of why it works as a literary device is because a common theme in fantasy is that names have power over things: if you want to imprison a demon or bind a dragon, Step One is usually to discover its true and secret name—either through trickery or scholarship—which you then weave into some incantation of warding or other. A name that inherently resists being pronounced by mortal tongue? That hints at dark and powerful things; things which a right-thinking person has no business even speaking of.
And I’m always reminded of this trope when I talk about phthalates. It’s a cursed and unnatural string of letters. Who the hell puts a “th” right after a “ph”? That sound combo is fine, I guess, at the end of a word like “fifth”…but to start a word like that? Deranged.
Just like in the fantasy novels, this is because these things were named in an arcane and hostile language: the language of chemistry. The root of the word comes to us from the ancient “naftu”, the name they called the black oil from the tar pits which were humanity’s first source of petroleum products. But as the compounds in that stuff were refined and transformed and derivatized to create new things, their names were as well: naftu becomes naphtha, which can be refined to naphthalene. Play around in that space, and you’ll find phenolphthalein (which, by a funny coincidence, is very useful as a pH indicator)…as well as the phthalates.

And here, again like in the fantasy novels, this thing with a difficult name is strange, powerful, fascinating—and sinister.
Phthalates are plasticizers, which is another way of saying that they’re the secret sauce behind plastic’s status as a “miracle material”. Plastics are able to take on a practically infinite variety of forms: from the tray your shitty microwaveable frozen lunch comes in, to the clear film on top that you poke holes in to let the steam vent, to the disposable fork you eat it with—and all the way to the trash bag you’ll throw it away in when the cooling cheese has congealed too much for you to deceive yourself into pretending it’s good anymore.
Better living through chemistry, eh folks?
We’ll save the bleakness for later, though; let’s talk about the science for a second. If you could zoom way in, down to a molecular level on a piece of plastic, you’d see something that looks basically like a clump of straw, or cat hair. Countless straight, slender chains of a chemical like PVC, all haphazardly and hopelessly interwoven with one another, so that moving any one bit moves the rest, causing the whole mess to behave like a singular, solid thing.
But the versatility of plastics lies in the fact that a “clump” doesn’t have to behave quite like a solid: it retains some of the noodly properties of its constituent parts, stretching and bending in ways that a more solid object couldn’t.
Those special properties depend largely on the interactions among those hairlike molecules. How hard is it to bend a clump of hair? The answer depends on a stunning array of variables, most of which are never the same twice: how many times is the average strand in this clump woven around other strands? How are they all oriented, and over what length scales? How much tension can the average strand undergo without breaking?
Change any of these things, and you can change the clump’s properties; massage some conditioner into it, and suddenly some spots that were previously friction points will start to slide by each other. Now you can stretch it a little more easily.
In this analogy, the molecular chains of PVC are the “hair”, while the plasticizers—the phthalates—are the conditioner. Add just a tiny dash of phthalates to your plastic, and you’ve got the thick, sturdy white PVC pipes that you find under a sink as part of a house’s standard plumbing. Add a little more, and you can get the thin but sturdy plastic that makes up the frame of a Mr. Coffee. Still more phthalate, and you’ve got the kind of flexible tubing used to make a garden hose, or the straw that makes a camelbak. Even things like plastic wrap, or the thin plastic peel-away film you find on most refrigerated goods is basically made by the same recipe.
With me so far? Okay, great—because the key thing about this model of plastics as “hair clump” + “conditioner” is that it makes it really easy to understand: There’s nothing holding it all together. The tangliness keeps the hairs more-or-less in place, and surface tension sticks the liquid to the hairs—but if you pick it up, your hand is gonna get wet.
Phthalates leach out extremely easily, is what I’m saying. They help give soft plastics their semi-fluid flexibility because they possess that fluidity for themselves, and they’re not as readily trapped in the matrix of the material as the long strandlike molecules. Ones on the surface of a material want to flow, to follow the concentration gradient from high to low, as all things do. If those surfaces are in contact with food—especially fatty or oily food—that’s where they’ll flow to. High temperatures, like the kind you get when you ladle a steaming scoop of chicken tikka masala into a disposable carryout tub, increase this rate of flow.
So: Why do we care?
More than once, I’ve tried to talk to someone about this one, only to be waved off with a “yeah, yeah, EVERYTHING gives you cancer.” And sure, a couple of the phthalates appear to be carcinogenic…but that’s not my main concern here.
Phthalates probably won’t kill you—they’ll just make you want to die.
The Suicide Molecule
Tryptophan is an amino acid that plays a big role in your neurochemistry: it’s how your body makes serotonin—and, in turn, melatonin. But those aren’t the only things tryptophan can turn into.
The pools of molecules in your cells behave a lot like water in pipes, as they get transformed from one thing into another by enzymes. The pipe that leads from tryptophan to serotonin only has so much capacity, so a good chunk of the tryptophan in your brain gets shunted down an alternative pipe, which leads to a molecule called kynurenine.
One of the offshoots from the kynurenine pool is a molecule called kynurenic acid. This is interesting in its own right because it’s what we call an NMDA antagonist—which means it’s in the same class of chemical as PCP or ketamine. We’ll talk more about that in a later piece, but for now let’s just say your brain doesn’t want too much of that around—so the pipe that leads to kynurenic acid is pretty narrow as well.
But there are two other pipes that branch off from kynurenine. One leads to a molecule called picolinic acid (which appears to play a role in helping you retain and transport metals like zinc), while the other leads to something called quinolinic acid. This is the interesting one.
Quinolinic acid is a neurotoxin. Specifically, an excitotoxin: it binds to a certain type of glutamate receptor and locks it in a state of continuous activation. Since glutamate is the main excitatory neurotransmitter in the brain, this does nasty things to the neuron that the receptor is attached to. It makes it hyperactive, firing over and over again until—like a lightbulb in a power surge—it burns out.
Now, this seems like a bit of a design flaw, doesn’t it? Why do we even have a metabolic pathway that ends up producing a powerful neurotoxin? The answer is that quinolinic acid isn’t a dead-end: another enzyme can take it and turn it into the B-vitamin niacin—a trick which is useful enough that it’s worth the risk of having a toxic intermediate in the process.
When everything is working as it should, the quinolinic acid in your brain is converted to niacin pretty much as soon as it’s produced, so it never has a chance to build up to toxic concentrations. If there’s a lot of kynurenine floating around, your cells produce more of the enzyme that converts kynurenine into picolinic acid. This acts like a safety valve, relieving the overpressure that would result in neurotoxic amounts of quinolinic acid being generated.
This is where some of the most common phthalates exert their toxicity. Some break the enzyme that leads to picolinic acid—clogging the safety valve that’s supposed to prevent too much kynurenine from turning into quinolinic acid. But phthalates are a diverse class of chemicals, and—as it happens—others break the enzyme that’s supposed to turn quinolinic acid into niacin and remove the neurotoxin once it’s been produced. If it’s surprising that these compounds have two distinct and independent ways of increasing the amount of quinolinic acid in your brain, take another look at the structures of the chemicals.

Now, I understand that this is all pretty abstract. Most of us know what a rush of dopamine or adrenaline feels like, but few have a reference point for “glutamatergic excitotoxicity”.
But, like dopamine, quinolinic acid is an endogenous, neuroactive compound. We’ve probably all felt it! The half life in a healthy brain is apparently on the order of 20 minutes.
How would we expect it to manifest, this toxic overstimulation of excitatory receptors Intrusive thoughts? Circular patterns of thinking that can’t be quelled? Compulsions? Tics?1
And how would it feel? What states of mind, or experiences, are associated with quinolinic acid, the way excitement and anxiety are associated with adrenaline?
Apparently: wanting to kill yourself.
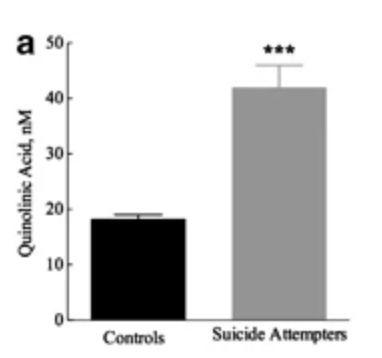
The above figure comes from an absolutely heroic study, where researchers made the most direct possible measurement of subjects’ brain chemistry by sampling their cerebrospinal fluid. They looked at quinolinic acid levels in the brains of people who’d just tried to commit suicide, and compared it to levels in healthy control brains. Suicide attempters had 2-3x as much in their brains as healthy individuals.
The link goes beyond acute suicidality, and appears to generalize to a finding of more quinolinic acid in certain brain regions of severely depressed people overall.
The parallels to a Lovecraftian ghoul really are strong, aren’t they? Victims of the things with unpronounceable names—they’re never found with the flesh rent from their bones by anything so mundane as vicious claws. Instead, the coroner’s inquest finds that the victim, after suffering from a long bout of insomnia, snapped and shot his family, then himself. The frantic scribblings in his journal, about protecting his family from the things closing in, the things worse than death…a tragic sign of his encroaching psychosis.
Measuring Taints
But to focus solely on phthalates’ neuropsychiatric effects would be to sell these miracle materials short. In animal experiments, in utero exposure to phthalates seems to induce “testicular dysgenesis syndrome”, while a ten-day regimen of exposure in pre-adolescent male mice is enough to disrupt sperm production and cause a reduced ano-genital distance (AGD) in males.
This is a bad sign. The process by which the genitals migrate forward during development is governed by androgens, so “taint length” is often used as an indicator of exposure to anti-androgenic compounds early in life. The studies in mice and rats which have shown concrete endocrine-disrupting effects are, of course, using much higher concentrations than what you’d expect most humans to be exposed to—but keep in mind that this is part of the point of animal studies; not to exactly replicate the circumstances of human exposure, but to exaggerate them so that subtle effects can be detected.
Other studies have attempted to assess the relationship between AGD and prenatal phthalate exposure in humans, but most of the ones I’ve found have used an “anogenital index”—which is just distance divided by weight—and which means that fatter babies would have lower numbers on average. Since dietary exposure to phthalates is likely associated with greater consumption of processed foods, I’m not impressed by this particular metric. Nevertheless, some have found significant associations between a mom’s urinary phthalate concentration and AGD itself.
Warding the Demon
Suffice to say we want to avoid these chemicals. How?
Phthalates are fat-soluble, and are found in plastics. So the obvious starting point is: avoid foods, especially fatty ones, that have been in contact with plastics for a long time or at a high temperature.
However, this is both nearly impossible in the modern world, and potentially not even that useful of a heuristic.
Why? The hard part isn’t the number of different foods in which phthalate residues have been found—it’s the fact that levels often vary wildly, without any apparent rhyme or reason. Consider the concentrations of DEHP, one of the most common ones, reported in a variety of cheeses:

It doesn’t look like there’s any kind of relationship between the fat content of the cheeses, the type of packaging, or how long they spend in packaging on shelves and their DEHP levels.2
In many cases, it seems levels have less to do with the packaging of the finished product, and more to do with processes “behind the scenes”. A predominant source of phthalates in dairy appears to be the plastics used in milking machines, and the tubes and bins that the milk is piped through on its way to becoming cheese etc. This tracks intuitively with the finding that the powdered cheeses used for boxed mac ‘n cheese contain about 4x the phthalates, per gram of fat, compared to real cheese.
But for every study that suggests a heuristic solution to avoiding phthalates, there’s one that makes it seem hopeless: a pretty comprehensive Belgian study found that bread, even fresh-baked at a local bakery and bagged in paper, is one of the higher-phthalate foods out there. Levels depended more on the bakery than anything else, although some of the contamination appears to have come from the flour.
Extra-virgin olive oils, which are extracted by mechanical pressing, generally have lower concentrations of phthalates than cheaper-grade versions like “olive pomace oil”, which are extracted from the leftovers of the first press using chemical solvents. Worse, some of the phthalates found in oils, like di-isobutyl phthalate (or DiBP), aren’t approved for use in food-contact materials.
This is a problem, to the extent that regulators aren’t totally asleep at the wheel here. They’ve used animal experiments to figure out how much DEHP you should be able to eat without it hurting your sperm quality,3 and they’ve passed laws requiring new food-contact materials to be checked in a lab, to show that they won’t leach more than a fraction of that amount under the conditions of expected use. If we don’t have data on how much of a certain phthalate should be safe to eat, it’s not allowed in food-contact material.
So how are these unapproved plasticizers making it into the food? The short answer is that they’re everywhere. Maybe the solvents used for olive oil extraction extraction come in plastic jugs. Maybe when you buy a big plastic vat to extract your olive dregs in, nobody asks what it’s for.
But as with our last entry in this series, this is a problem of a system with too many moving parts. There’s a great example of this in a paper from 1989, where they found that chocolates, potato chips, and a number of other common snack foods had substantial levels of phthalates in them—despite the fact that they were wrapped in polypropylene film, which should contain none of these chemicals. But here, the source of these mystery phthalates turned out to be the packaging after all: specifically, the inks used to print the bags and wrappers. This wouldn’t have been a problem (since the printing was only on the outside) except that those wrappers were printed into long, spooled rolls—meaning the ink on the outside of each layer would sit sandwiched against the inside surface of the bag in the next layer on the roll. Phthalates leached from ink to the adjacent inner surface, then from that surface back into the food, once it was packed.
This is the point at which any reasonable person will understandably throw their hands up and say “What is the point in trying?”. Even if you were to become the kind of person who carefully inspects the little numbers on the bottom of plastic containers, who learns to recognize polypropylene vs. PVC plastic, so as to avoid the phthalates in the latter, something like the ink/spool effect can still crop up and ruin your efforts. This is an issue of trying to know too much—of trying to engineer solutions to a problem that is already the result of too much engineering. Ironically, the person who doesn’t know the difference between polypropylene and PVC, but who just knows “avoid plastic x food”, will be spared that exposure.
Still, the modern world is so plasticized that it’s impossible to totally avoid it—so the mental calculation I do when ranking preferences involves trying to minimize a quantity that looks something like:
[(food surface area in contact with plastic) x (flexibility of plastic) x (fattiness of food) x (time) x (temp)].
So I buy my oils in glass, and try to find the least processed version of a thing. I don’t do frozen meals, and I avoid using condiment packets of any kind—but particularly mayonnaise. When I unwrap a new block of cheese, I’ll scrape away the shiny outer layer with a sharp knife, then rewrap it in foil to store it in the fridge. The top layer of a pint of ice cream, which sat in contact with the clear plastic film, gets shaved away and washed into the sink.
Does any of this help? I don’t know. I wish I had better news or better solutions for you here; it’s a bummer to say “there are chemicals in your food that might make you want to kill yourself, and there’s no foolproof way to avoid them.”
But maybe even that knowledge is a comfort to some people, as strange as it sounds. When a demon whispers in your ear, telling you—in a voice that sounds a lot like your own—that it’ll never get better, that you should end it…maybe it’s enough to know that its voice is not yours.
There is power in the names of things.
—🖖🏼💩
Curiously enough, quinolinic acid administered to animals causes similar brain lesions to those seen in Huntington’s chorea, a genetic disease which is associated with serious depression and cognitive changes in early stages before progressing to uncontrollable muscle spasms.
Plastic wraps are now made using Diethyl hexyl adipate (DEHA) as a plasticizer, which has been found in clingfilm-wrapped cheeses at up to a whopping 429 mg/kg. This compound appears not to have the same hormonal effects as phthalates, but I’m still not sure I trust it on those merits alone.
It’s worth noting here that the hormone-disrupting effects of phthalates appear to be totally independent of their effect on quinolinic acid levels, but these hormonal effects are the primary factor under consideration when regulators decide how much of these compounds should be allowed in food. That is to say: the amount of phthalate exposure it takes to increase a person’s risk of suicide could be much lower than the amount it takes to disrupt their hormone levels. We simply don’t know.








Thank you for this piece! Also love your writing style as well and it reminds me of derrangedphysiology blog which might be of interest. Looking forward to the next writings!!
Grape seed https://www.sciencedirect.com/science/article/pii/S1756464620300852